What is a Redline?
A redline document shows additions, deletions and other content and formatting changes between the active standard and the previous edition.
If you purchase a redline upgrade, you will receive TWO versions of the document – the current edition of the standard and the redline version.
How to Buy
To upgrade your document to include the redline version:
- Add the document to your cart.
- Select the Upgrade with Redline option in the cart pop up.
- Click “Add Selected Upgrades” button to add the redline version to your cart.
What is a Multi-User PDF?
Multi-User PDF files allow you to purchase access to specific documents for use in your company for up to nine users.
How to Buy
To upgrade your PDF to a Multi-User version:
- Add the PDF to your cart.
- Select your desired number of users.
- Click "Add Selected Upgrades" button to add the multi-user version of the PDF to your cart.
Upon completing your order, you’ll be prompted to enter the email addresses of the users who will need access to the PDF.
What is a Multi-User Redline PDF?
A redline Multi-User document gives you the both the ability to compare all the changes between the active standard and the previous version and to provide you with access for up to nine users.
Use Redlines To:
-
Identify updates in minutes, not hours
-
Effortlessly implement changes to procedures, equipment and products
-
Save time and resources
-
When you purchase Techstreet redlines, you receive TWO documents -- the clean, active version of the standard and the redline version.
Use Multi-User PDFs To:
- Save money when you buy a document for your team
- Save time by purchasing once for multiple users
- Effortlessly distribute a PDF to your team with our self-service interface
How to Buy
- To upgrade your PDF to a Multi-User Redline version, simply add the PDF to your cart and you will be presented with a pop-up window that will display user and pricing information.
- From the pop-up window, select your upgrade and press the “Add Selected Upgrades” button.
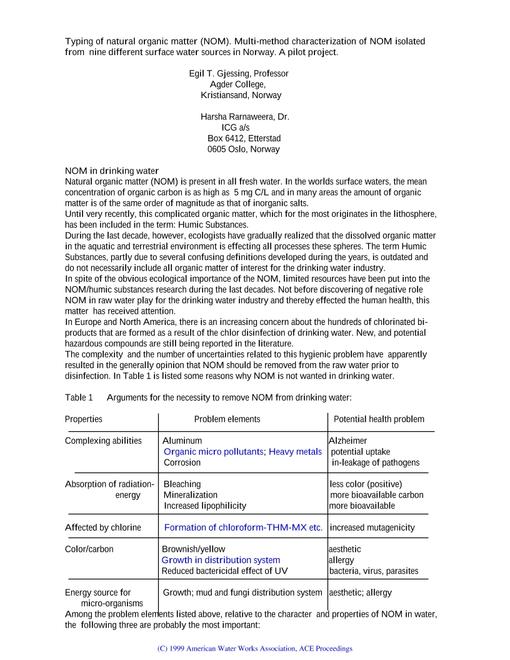
There are corrections to this edition, which are available as separate documents.
There are amendments to this edition, which are available as separate documents.
Techstreet's Printed Edition + PDF option allows you to purchase a print edition of your document along with a PDF for immediate download at a package price.
This is a historical version of this document. A more current edition is available.
This is the most up-to-date edition of this document.
A secure PDF contains features that enforce existing copyright laws by preventing reproduction or distribution to other users. In order to successfully use your secure PDF, Adobe Acrobat Reader 8.0 or higher, and the free FileOpen plug-in installed.